Medical
Trusted Global Leader
Complete Industrial Chain
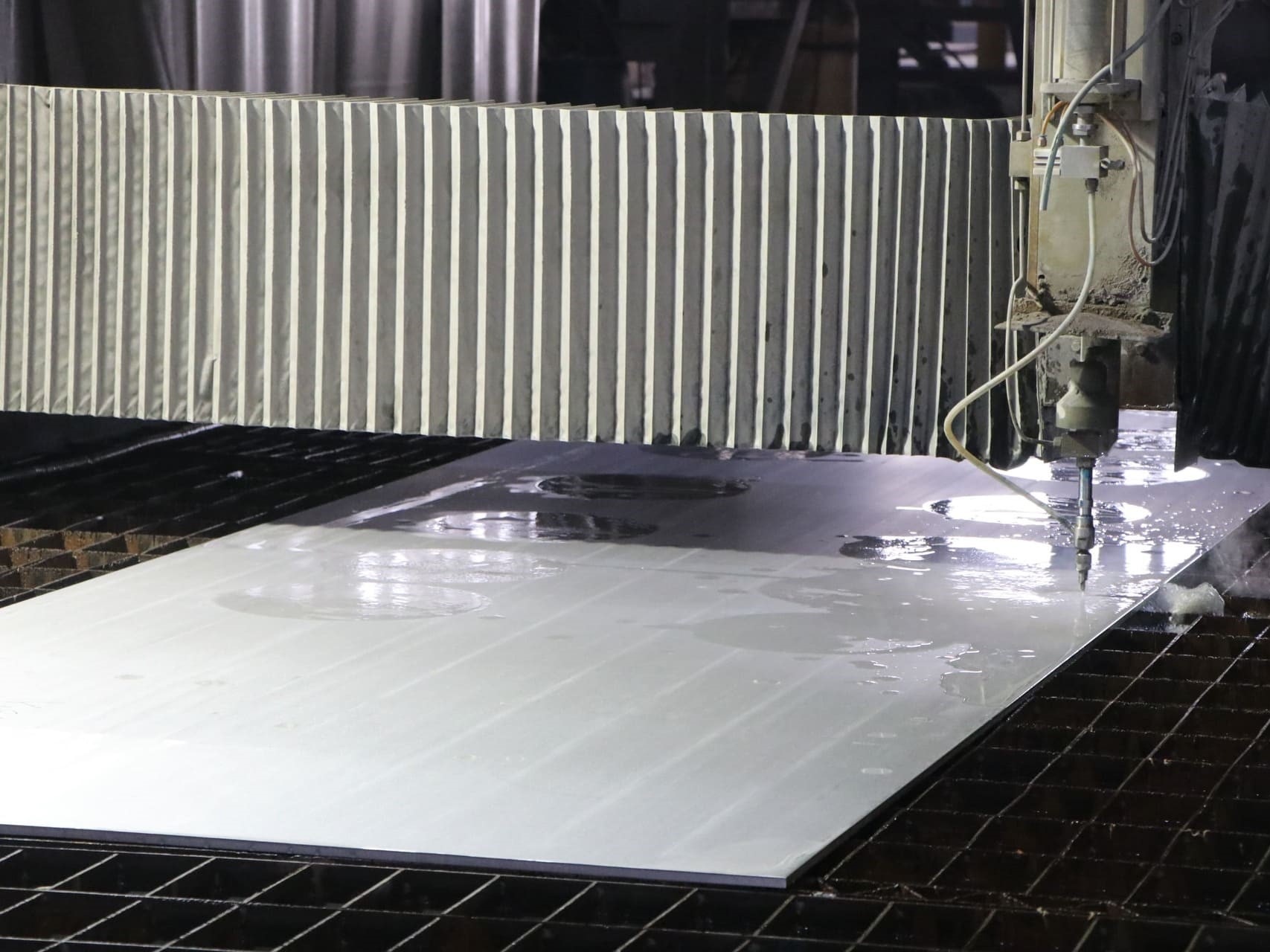
Advanced Equipment and Reliable Capacity
JH has built a complete titanium processing chain — from raw material sourcing to precision forming and quality inspection. Backed by rich titanium resources and a stable supply of high-purity ingots, the company ensures consistent quality and fast delivery. Its advanced facilities include vacuum degassing furnaces, rolling ring and fast-forging units, laser cutting systems, annealing and pit-type furnaces, and precision grinding equipment. These technologies enable JH to produce medical-grade titanium materials with exceptional surface finish, mechanical stability, and biocompatibility, meeting the strict standards required for orthopedic, dental, and surgical applications, while also serving select industrial needs.



Premium Medical-Grade Titanium Raw Materials
Why Choose JH



Comprehensive Guide to Medical Titanium: Properties, Grades, Applications, and Manufacturing Process
Executive summary: Medical-grade titanium and titanium alloys are favored for implants and devices due to high strength-to-weight ratio, corrosion resistance, and excellent biocompatibility. This guide explains properties, grades/standards, manufacturing, surface treatments, applications, and regulatory/testing considerations.
1. Key properties
- Physical & mechanical: High strength-to-weight; modulus closer to bone than many metals; good fatigue strength.
- Chemical: Excellent corrosion resistance from stable TiO2 passive film.
- Biological: High biocompatibility and strong osseointegration with appropriate surface treatment.
- Processing: Suitable for forging, machining, and additive manufacturing (SLM/EBM).
2. Common medical titanium grades and standards
Commercially pure titanium (cp-Ti, Grades 1–4) — increasing strength from Grade 1 to Grade 4. Used for dental components and some implants.
Ti-6Al-4V (Ti64) — high-strength alloy widely used in orthopedics and trauma fixation. Ti-6Al-4V ELI is the implant-grade variant.
Other alloys: Ti-6Al-7Nb and beta-type alloys (e.g., Ti-13Nb-13Zr) for specific applications.
Standards (examples): ASTM F67 (cp-Ti), ASTM F136 (Ti-6Al-4V ELI), ISO 5832 series.
3. Clinical applications
- Orthopedics: joint components, plates, screws, intramedullary nails, spinal implants.
- Dental: endosseous implants, abutments.
- Surgical instruments and implant housings for electronics.
- Maxillofacial and cranial plates/meshes.
4. Manufacturing process
- Raw material & melting: VIM, VAR, EBM to produce high-purity ingots; powder atomization for AM.
- Hot working: forging, rolling, extrusion to refine microstructure.
- Machining: precision CNC (careful tooling & cooling needed).
- Additive manufacturing: SLM/EBM for complex & porous geometries; follow with HIP and heat treatment.
- Heat treatment: stress relief, solution & aging treatments for alloys when needed.
- Surface finishing: blasting, acid etch, anodizing, coatings (HA, TiN), laser texturing.
- Cleaning & sterilization: ultrasonic cleaning, passivation, validated sterilization (autoclave, EO, gamma).
5. Testing, quality control & regulatory
- Mechanical tests: tensile, fatigue, hardness.
- Corrosion testing in simulated body fluids.
- Biocompatibility per ISO 10993 series.
- Surface characterization: roughness, oxide thickness, SEM.
- Traceability and material certification; regulatory submissions (FDA, MDR, etc.).
6. Design & clinical considerations
- Minimize stress shielding via design and material choices.
- Optimize surface for osseointegration; control roughness and chemistry.
- Avoid unfavorable dissimilar-metal contacts; watch for fretting/corrosion at modular interfaces.
- Assess MRI compatibility and artifact behavior.
7. Emerging trends
- AM-enabled patient-specific implants and porous architectures.
- Biofunctional coatings and antimicrobial surfaces.
- Development of lower-modulus beta alloys for better bone matching.
8. Practical checklist
- Select appropriate grade/standard for application.
- Specify mechanical and fatigue requirements.
- Define surface finish and biological goals.
- Choose manufacturing route and arrange postprocessing.
- Prepare documentation and validation plan for regulatory submission.
For implementation help (material selection, process flow, or drafting regulatory documentation), tell me the specific implant or device you are designing and I’ll produce an action plan and suggested spec sheet.