Titanium Alloy For Medical Purposes
 Joints |
 Spinal Correction |
 Dental Implant |
 Medical Equipment |
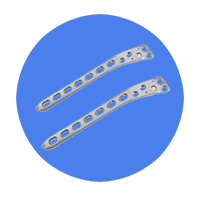 Bone Plate |
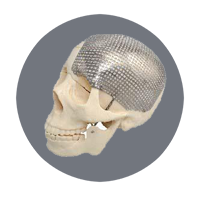 Artificial Skull |
 Bone Nail |
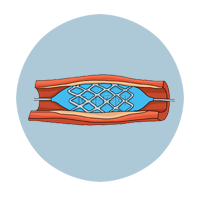 Heart Stent |
JH, serving the medical industry since 2009
that meets the internationally recognised standards including ASTM F67 and ASTM F136.
-
ASTM F67: This standard specifies the requirements for unalloyed titanium (Grades 1, 2, 3, and 4) used in surgical implants. It covers the chemical composition, mechanical properties, and permissible impurities for titanium intended for medical applications.
-
ASTM F136: This standard applies to titanium alloy (Ti-6Al-4V) and specifies requirements for chemical composition, mechanical properties, and microstructure. It is commonly used for surgical implants due to its strength and biocompatibility.
All medical products are certified and supplied with a CE mark to indicate that the titanium conforms to health, safety and environmental protection standards for products sold into the European Economic Area (EEA)
Titanium is widely used in the medical industry due to its biocompatibility, corrosion resistance, and strength. Here are some of its primary applications:


